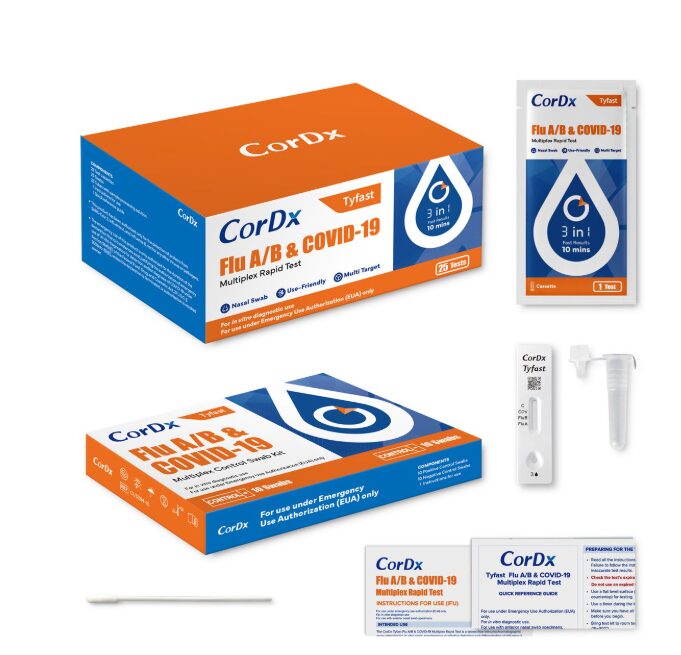
- Kit Contents: (25) Test Cassettes, (25) Nasal Swabs, (25) Tubes with Sample Processing Solution, Instructions For Use, Quick Reference Guide
- For use under Emergency Use Authorization (EUA) only
- For in vitro diagnostic use
- For use with anterior nasal swab specimens
- Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. § 263a, that meet the requirements to perform moderate, high or waived complexity tests.
- This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
CorDx Tyfast Flu A/B & COVID-19 Multiplex Rapid Test, FDA Emergency Use Authorization (25 Tests/Kit) New
- SKU: 12345
- The CorDx TyFast Flu A/B & COVID-19 Multiplex Rapid Test is a lateral flow immunoassay intended for the qualitative detect and differentiate of SARS-CoV-2 and influenza A and B antigens in nasal samples (from both nostrils). The test provides an efficient and accurate diagnostic solution in just 10 minutes, giving people fast way to prioritize their health.
- In Stock
$ 323
Buy 10 or more for
$ 300/box
Buy 20 or more for
$ 250/box
Buy 25 or more for
$ 200/box
- Details
- Specifications
- Alternatives